Home
Nanomaterials and Catalysis
Multiscale Modeling of Bifunctional Catalysts for the Water-Gas Shift Reaction
For heterogeneously catalyzed reactions with more than one key surface intermediate, it is likely that multiphase catalysts have a significant advantage over conventional monophase catalysts since each phase can potentially be adjusted independently to activate a key reaction step. At the same time, our understanding of bifunctional multiphase systems is relatively poor. It is the objective of this research program to significantly enhance our molecular understanding of heterogeneous catalysis at the three-phase boundary (TPB) of a gas-phase, a reducible oxide surface, and a noble metal cluster. To enable this theoretical investigation of chemical reactions at the TPB, we propose to develop and validate a highly efficient and accurate computational strategy for these systems. It is our firm belief that only with a more accurate computational multiscale strategy that permits the reliable investigation of reactions on strongly correlated reducible oxide surfaces and metal clusters, will it be possible to truly understand the nature of the active sites, the origin of catalytic activity, and the reaction mechanism at the TPB under reaction conditions. As a model system for our computational study, we investigate the water-gas shift (WGS) reaction on titania and ceria supported mono- and bimetallic clusters of Au and Pt.
Selected References:
"Understanding the Nature and Activity of Supported Platinum Catalysts for the Water-Gas Shift Reaction: From Metallic Nanoclusters to Alkali-Stabilized Single Atom Cations," S.C. Ammal, A. Heyden, ACS Catalysis 9, 7721-7740 (2019).
"Identifying active sites of the water-gas shift reaction over titania supported platinum catalysts under uncertainty," M.A. Walker, D. Mitchell, G.A. Terejanu, A. Heyden, ACS Catalysis 8, 3990-3998 (2018).
"Titania-supported single-atom platinum catalyst for water-gas shift reaction," S.C. Ammal, A. Heyden, Chem. Ing. Tech. 89, 1343-1349 (2017).
"Water-gas shift activity of atomically dispersed cationic platinum versus metallic platinum clusters on titania supports," S.C. Ammal, A. Heyden, ACS Catalysis 7, 301-309 (2017).
"Uncertainty quantification framework applied to the water-gas shift reaction over Pt-based catalysts," E. Walker, S.C. Ammal, G. Terejanu, A. Heyden, J. Phys. Chem. C 120, 10328-10339 (2016).
"Water-Gas Shift Catalysis at Corner Atoms of Pt Clusters in Contact with a Pt/TiO2 (110) Support Surface," S.C. Ammal, A. Heyden, ACS Catalysis 4, 3654-3662 (2014).
"On the Importance of the Associative Carboxyl Mechanism for the Water-Gas Shift Reaction at Pt/CeO2 Interface Sites," S. Aranifard, S.C. Ammal, A. Heyden, J. Phys. Chem. C 118, 6314-6323 (2014).
"On the Importance of Platinum-Ceria Interfaces for the Water-Gas Shift Reaction," S. Aranifard, S. C. Ammal, A. Heyden, J. Catal. 309, 314-324 (2014).
"Origin of the Unique Activity of Ptn/TiO2 Catalysts for the Water-Gas Shift Reaction," S.C. Ammal, A. Heyden, J. Catal. 306, 78-90 (2013).
"Modeling the noble metal/TiO2 (110) interface with hydrid DFT functionals: A periodic electrostatic embedded cluster model study," S.C. Ammal, A. Heyden, J. Chem. Phys. 133, 164703 (2010).
Science Based Nano-Structure Design and Synthesis of Heterogeneous Functional Materials for Energy Systems
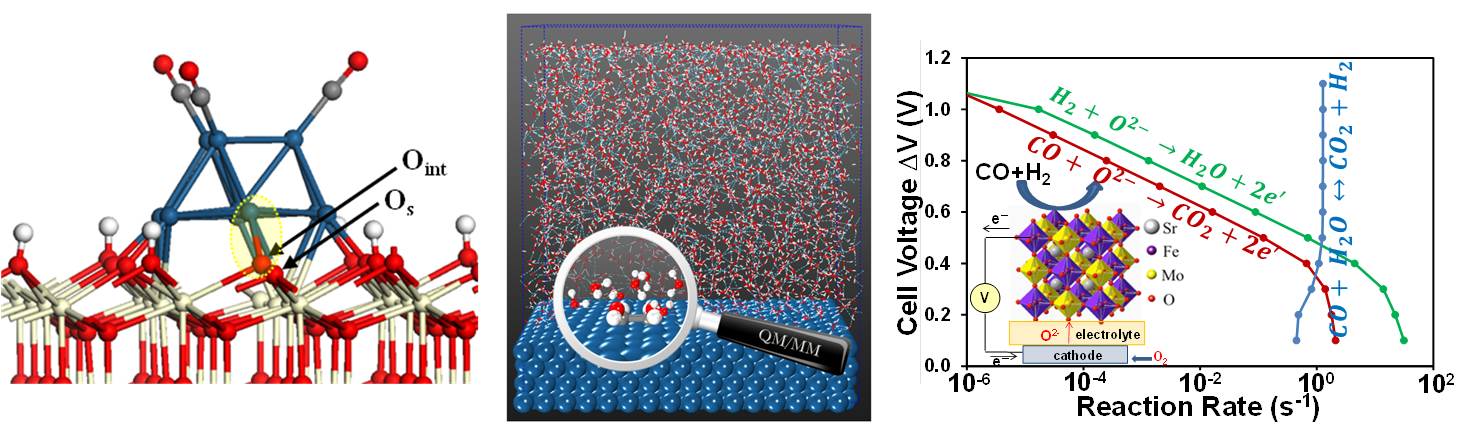
Our primary task within this "HeteroFoam" EFRC is the rational design of sulfur and carbon tolerant anode materials for solid oxide fuel cells. In particular, we are interested in doped perovskites and heterogeneous materials containing multiple phases such as Ni/YSZ. Furthermore, we aim at developing novel multiscale models for electrochemical systems that permit the design and discovery of novel materials.
Selected References:
"Reaction Kinetics of the Electrochemical Oxidation of CO and Syngas Fuels on a Sr2Fe1.5Mo0.5O6 Perovskite Anode," S. C. Ammal, A. Heyden, J. Phys. Chem. C 118, 23545-23552 (2015).
"Mechanism of Sulfur Poisoning of Sr2Fe1.5Mo0.5O6 Perovskite Anode under Solid Oxide Fuel Cell Conditions," E. Walker, S. C. Ammal, S. Suthirakun, F. Chen, G. A. Terejanu, A. Heyden, J. Phys. Chem. C 118, 23545-23552 (2014).
"Theoretical Investigation of H2 Oxidation on the Sr2Fe1.5Mo0.5O6 (001) Perovskite Surface under Anodic Solid Oxide Fuel Cell Conditions," S. Suthirakun, S. C. Ammal, A. B. Munoz-Garcia, G. Xiao, F. Chen, H.-C. zur Loye, E. A. Carter, A. Heyden, J. Am. Chem. Soc. 136, 8374-8386 (2014).
"Combined DFT and Microkinetic Modeling Study of Hydrogen Oxidation at the Ni/YSZ Anode of Solid Oxide Fuel Cells," S. C. Ammal, A. Heyden, J. Phys. Chem. Lett. 3, 2767-2772 (2012).
"Obtaining mixed ionic/electronic conductivity in perovskite oxides in a reducing environment: A computational prediction for doped SrTiO3," S. Suthirakun, S. C. Ammal, G. Xiao, F. Chen, K. Huang, H.-C. zur Loye, A. Heyden, Solid State Ionics 228, 37-45 (2012).
"Density Functional Theory Study on the Electronic Structure of n- and p-type doped SrTiO3 at Anodic Solid Oxide Fuel Cell Conditions," S. Suthirakun, S. C. Ammal, G. Xiao, F. Chen, H.-C. zur Loye, A. Heyden, Phys. Rev. B 84, 205102 (2011).
